5) The volume of several aqueous copper sulfate (CuSO4) solutions was precisely measured at SATP. All of the solutions were made by adding copper sulfate to 100.00 g of water. ncopper sulfate (mol) Vsolution (mL) 0 100.18 0.0330 100.16 0.0696 100.36 0.111 100.81 0.156 101.62 First, estimate the concentration-dependent partial molar volume of copper sulfate in water by taking finite differences. Second, fit the data to a hyperbola and take a derivative of the hyperbola to accurately calculate the partial molar volume of copper sulfate in water at each concentration. Interpret the partial molar volume at infinite dilution - what must copper sulfate be doing to the nearby water molecules?
5) The volume of several aqueous copper sulfate (CuSO4) solutions was precisely measured at SATP. All of the solutions were made by adding copper sulfate to 100.00 g of water. ncopper sulfate (mol) Vsolution (mL) 0 100.18 0.0330 100.16 0.0696 100.36 0.111 100.81 0.156 101.62 First, estimate the concentration-dependent partial molar volume of copper sulfate in water by taking finite differences. Second, fit the data to a hyperbola and take a derivative of the hyperbola to accurately calculate the partial molar volume of copper sulfate in water at each concentration. Interpret the partial molar volume at infinite dilution - what must copper sulfate be doing to the nearby water molecules?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.106QE: In the 1986 Lake Nyos disaster (see the chapter introduction), an estimated 90 billion kilograms of...
Related questions
Question
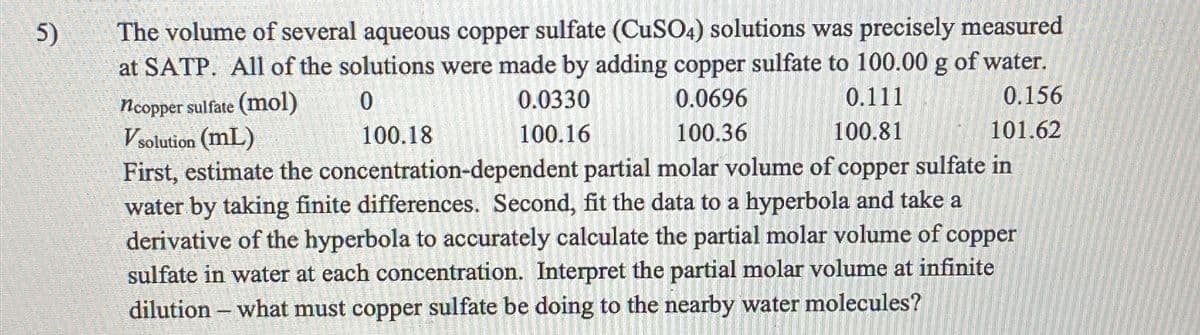
Transcribed Image Text:5)
The volume of several aqueous copper sulfate (CuSO4) solutions was precisely measured
at SATP. All of the solutions were made by adding copper sulfate to 100.00 g of water.
ncopper sulfate (mol)
Vsolution (mL)
0
100.18
0.0330
100.16
0.0696
100.36
0.111
100.81
0.156
101.62
First, estimate the concentration-dependent partial molar volume of copper sulfate in
water by taking finite differences. Second, fit the data to a hyperbola and take a
derivative of the hyperbola to accurately calculate the partial molar volume of copper
sulfate in water at each concentration. Interpret the partial molar volume at infinite
dilution - what must copper sulfate be doing to the nearby water molecules?
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning