1. Write the RHR, OHR, and the balanced overall reaction of the voltaic cell. 2. Prove that the reaction in the voltaic cell is spontaneous by calculating E°cel using Eqn 11b-1 and the values listed in Table S1 of the Supplementary Material. 3. Calculate the theoretical Ecel using the Nernst Equation (Eqn 11b-2). 4. Compare the experimental Ecel from Table 11b-1 to the theoretical Ecel by calculating percent error using Eqn 11b- 4. experimental value - literature value % error = x 100 (Eqn 11b-4) literature value
1. Write the RHR, OHR, and the balanced overall reaction of the voltaic cell. 2. Prove that the reaction in the voltaic cell is spontaneous by calculating E°cel using Eqn 11b-1 and the values listed in Table S1 of the Supplementary Material. 3. Calculate the theoretical Ecel using the Nernst Equation (Eqn 11b-2). 4. Compare the experimental Ecel from Table 11b-1 to the theoretical Ecel by calculating percent error using Eqn 11b- 4. experimental value - literature value % error = x 100 (Eqn 11b-4) literature value
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question
100%

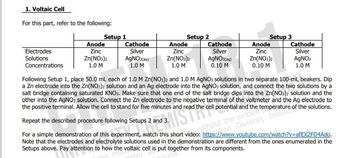
Transcribed Image Text:1. Voltaic Cell
For this part, refer to the following:
Setup 2
Anode
Setup 1
Setup 3
Anode
Cathode
Cathode
Anode
Cathode
Electrodes
Zinc
Silver
Zinc
Silver
Zinc
Silver
AGNO3(aqg)
0.10 M
Zn(NO3)2
0.10 M
Solutions
Zn(NO3)2
1.0 M
AGNO3(aq)
1.0 M
Zn(NO:)2
1.0 M
AGNO3
1.0 M
Concentrations
Following Setup 1, place 50.0 mL each of 1.0 M Zn(NO3)2 and 1.0 M AGNO3 solutions in two separate 100-mL beakers. Dip
a Zn electrode into the Zn(NO3)2 solution and an Ag electrode into the AGNO3 solution, and connect the two solutions by a
salt bridge containing saturated KNO3. Make sure that one end of the salt bridge dips into the Zn(NO3)2 solution and the
other into the AGNO3 solution. Connect the Zn electrode to the negative terminal of the voltmeter and the Ag electrode to
the positive terminal. Allow the cell to stand for five minutes and read the cell potential and the
Repeat the
procedure following Setups 2 and 3.
saton Division, Institue
For a simple demonstration of this experiment, watch this short video: https://www.youtube.com/watch?v=DafEX2FD4Ado.
Note that the electrodes and electrolyte solutions used in the demonstration are different from the ones enumerated in the
Setups above. Pay attention to how the voltaic cell is put together from its components.
nuna.

Transcribed Image Text:A. VOLTAIC CELL
1. Write the RHR, OHR, and the balanced overall reaction of the voltaic cell.
Prove that the reaction in the voltaic cell is spontaneous by calculating E°cell using Egn 11b-1 and the values listed
in Table S1 of the Supplementary Material.
3. Calculate the theoretical Ecell using the Nernst Equation (Eqn 11b-2).
4. Compare the experimental Ecell from Table 11b-1 to the theoretical Ecell by calculating percent error using Egn 11b-
4.
2.
experimental value - literature value
% error =
x 100
(Eqn 11b-4)
literature value
Note: Steps 3 and 4 should be done for EACH setup.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Solutions and Answers from numbers 2-3

Transcribed Image Text:A. VOLTAIC CELL
1. Write the RHR, OHR, and the balanced overall reaction of the voltaic cell.
2. Prove that the reaction in the voltaic cell is spontaneous by calculating Eºcell using Eqn 11b-1 and the values listed
in Table S1 of the Supplementary Material.
3. Calculate the theoretical Ecell using the Nernst Equation (Eqn 11b-2).
4. Compare the experimental Ecell from Table 11b-1 to the theoretical Ecell by calculating percent error using Eqn 11b-
4.
% error =
experimental value - literature value
literature value
x 100
(Eqn 11b-4)
Note: Steps 3 and 4 should be done for EACH setup.
Transcribed Image Text:1. Voltaic Cell
For this part, refer to the following:
Anode
Anode
Electrodes
Anode
Zinc
Zn(NO3)2
Cathode
Silver
AgNO3(aq)
Cathode
Silver
Solutions
Concentrations
Cathode
Silver
AgNO3(aq)
0.10 M
Zinc
Zn(NO3)2
1.0 M
Zinc
Zn(NO3)2
AgNO3
1.0 M
1.0 M
0.10 M
1.0 M
Following Setup 1, place 50.0 mL each of 1.0 M Zn(NO3)2 and 1.0 M AgNO3 solutions in two separate 100-mL beakers. Dip
a Zn electrode into the Zn(NO3)2 solution and an Ag electrode into the AgNO3 solution, and connect the two solutions by a
salt bridge containing saturated KNO3. Make sure that one end of the salt bridge dips into the Zn(NO3)2 solution and the
other into the AgNO3 solution. Connect the Zn electrode to the negative terminal of the voltmeter and the Ag electrode to
the positive terminal. Allow the cell to stand for five minutes and read the cell potential and the
the solutions.
Repeat the described procedure following Setups 2 and
Step 2
ted without the written temperature of
Division,
For a simple demonstration of this experiment, watch this short video: https://www.youtube.com/watch?v=afEX2FD4Ado.
Note that the electrodes and electrolyte solutions used in the demonstration are different from the ones enumerated in the
Setups above. Pay attention to how the voltaic cell is put together from its components.
to how the voltaic cell is put toget
Setup 1
Setup 2
Setup 3
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning