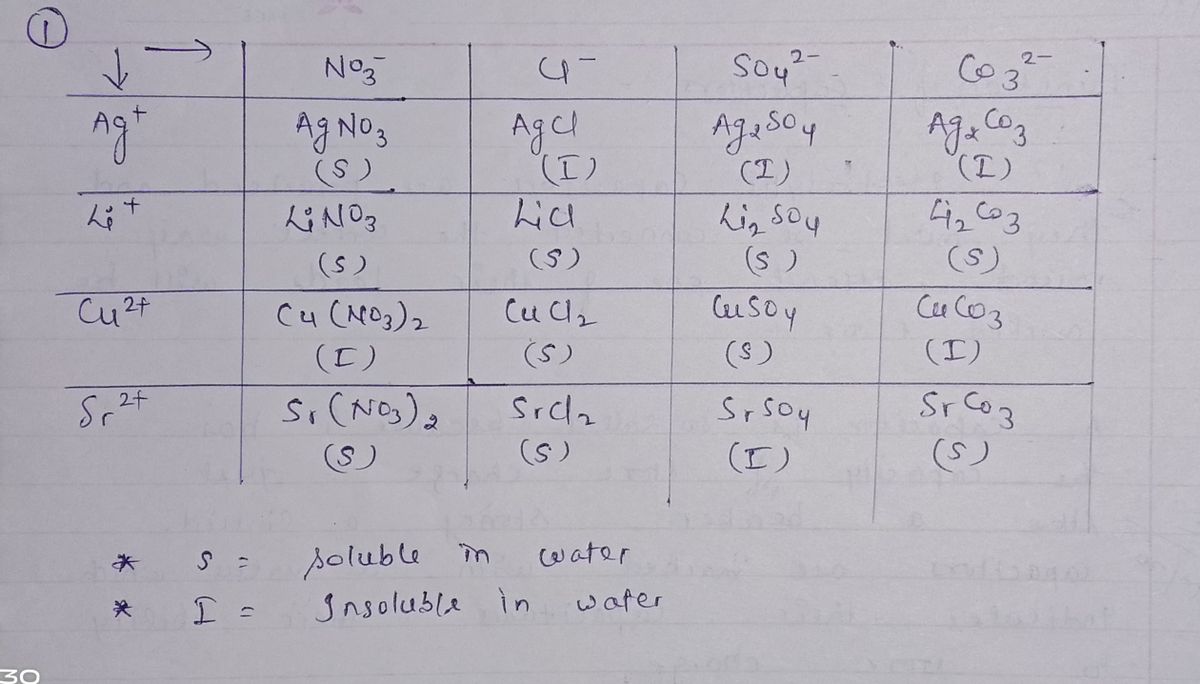
1. Complete the following solubility table. (Simply enter S for soluble or I for insoluble in each cell). NO; Li* Ag" Cu²" Sr* 2. Write net ionic equations for those combinations that produce insoluble products. Ag + Co
1. Complete the following solubility table. (Simply enter S for soluble or I for insoluble in each cell). NO; Li* Ag" Cu²" Sr* 2. Write net ionic equations for those combinations that produce insoluble products. Ag + Co
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.52PAE: Because barium sulfate is opaque to X-rays, it is suspended in water and taken internally to make...
Related questions
Question
100%

Transcribed Image Text:14:52 Wed Feb 17
* 75%
T O
+ :
Name:
Date:
EXPERIMENT 2
QUALITATIVE CATION ANALYSIS
PRE-LABORATORY QUESTIONS
The following preparatory questions should be answered before coming to lab. They are intended
to introduce you to several ideas that are important to aspects of the experiment. You must turn
in your work to your instructor before you will be allowed to begin the experiment.
1. Complete the following solubility table. (Simply enter S for soluble or I for insoluble in
each cell).
NO3
SO2-
CO3²-
Cl
Li*
Ag*
I
Cu2+
Sr+
2. Write net ionic equations for those combinations that produce insoluble products.
Co
1
3
СHM 1024 — Ехрeriment 2. Qualitative Cation Analysis
Page 1 of 2
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning