
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.15B, Problem 12.10P
Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar but distinctive mass spectra.
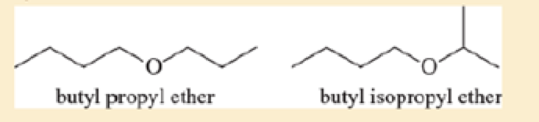
Both compounds give prominent peaks at m/z 116, 73, 57, and 43. But one compound gives a distinctive strong peak at 87, and the other compound gives a strong peak at 101. Determine which compound gives the peek at 87 and which one gives the peak at 101. Propose fragmentations to account for the ions at m/z 116, 101, 87, and 73.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the structure of the compound using the information below. Kindly show what information are obtained for each spectra which may help in predicting the structure.
From IR Spectra:
See the attached picture
From Mass Spectra
M+ = 87
From Proton and Carbon NMR Spectra
See attached picture.
A compound contains only C, H
and O. The mass spectrum shows
peaks at m/z = 58 and 43. What is the
structure of the compound?
Identify each of the following compound (C4H8O2) from its molecular formula and its IR and 1H NMR spectra: Note: Offset means: “add “offset to 8 ppm.”Assign the peaks in NMR spectrum. You must indicate clearly the hydrogen/s of the molecule responsible for the peak/s.
Cannot write only the structure
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar butdistinctive mass spectra.O Obutyl propyl ether butyl isopropyl etherBoth compounds give prominent peaks at m>z 116, 73, 57, and 43. But one compoundgives a distinctive strong peak at 87, and the other compound gives a strong peak at 101.Determine which compound gives the peak at 87 and which one gives the peak at 101.Propose fragmentations to account for the ions at m>z 116, 101, 87, and 73.arrow_forwardA compound displays key peaks at 1724, 1600, and 1585 cm-1 in its IR spectrum. There are also 2 peaks between 2700-2850 cm-1. The mass spectrum has a molecular ion with m/z 120. The base peak is at m/z=91. Draw a structure that best fits this data.arrow_forwardAn unknown organic compound is isolated. The compound produces a molecular ion at m/z = 101. There is no significant M+2 peak. The IR spectrum shows peaks ~2950 cm'. It does not have any peaks above 3000, nor around 1600-1800 or 1050 cm1. The 'H-NMR and 13C-NMR spectra are shown below. What is the name of this compound (IUPAC and common name are both acceptable)? 3H 2H PPM 20 10 30 PPM 50 40arrow_forward
- Which of these choices best describes the interpretation of the following peak that may be recorded in a 'H NMR spectrum? 1.38 & (1H, heptet (7 lines)). The underlined hydrogen atom is intended to be the one producing the peak that we are interpreting. More than one choice may describe the peak very well. O (CH3)2-CH-o (CH3)2-CH-C=0 (CH3)2-CH-C=C O (CH3)2-CH-N O (CH3)2-CH-C (CH3)2-CH-Ar None of these interpretations describes this peak.arrow_forwardA compound has the molecular formula of C3HgO. It exhibits 4 peaks in its 1H NMR spectrum. These peaks are (in 8): 3.6 (2H, t). 2.3 (1H, broad), 1.6 (2H, sextet). 1.05 (3H. t). Which of the following compounds is consistent with this data? 0 0 OMe OH OH O H.C-O-CH. О OH Caarrow_forwardFollowing are the 'H and 13C NMR spectra for each of four isomeric bromoalkanes with formula C4H9B.. Assign a structure to each pair of spectra and assign all H's and C's. Carbon spectrum C,H,Br CDCI3 85 80 75 70 65 60 55 50 45 35 30 15 10 5 Proton spectrum D C,H,Br 2.1 2.0 1.9 1.82 0.93 5.37 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0arrow_forward
- 3. Determine the structure of a compound with a molecular formula of C6H12O2 and exhibits the following 'H NMR spectrum. No peaks exchange in D20. The °C NMR has a peak at 170 ppm. Each red box indicates a single integration value (i.e., the peak at 4.05 ppm has an integration = 2). The signal at 2.05 ppm is a singlet. 11 15 10 9 7 6. 5 4 3. 6 (ppm) frequencyarrow_forwardA mass spectrum has an M+ peak at 170; and additional peaks at 127, 43, 29 and 15. Which of the following compounds could have produced this mass spectrum? iodoethane 2-iodopropane 1-chlorobutane 3-chloropentatnearrow_forwardA compond has a molecular ion peak at M/Z = 72 in the mass spectrum. It has a sharp peak around 1730cm-1 in the IR. What is the molecular formula of the compound and how many unsaturation is present in this compound?arrow_forward
- Butanal and methylpropanal give slightly different mass spectra. Both give a molecular ion peak at m/z = 72, but butanal gives four other peaks whereas methylpropanal only gives three. State the species responsible for the four other peaks in the mass spectrum of butanal and write equations to show their formation from the molecular ion.arrow_forward[References] The mass spectrum of compound A shows the molecular ion at m/z 85, an M+ 1 peak at m/z 86 of approximately 6% abundance relative to M, and an M+ 2 peak at m/z 87 of less than 0.1% abundance relative М. Assuming that compound A has only C, H, and one N atoms, determine the molecular formula, and then draw a possible structure if compound A has IR absorption at 1620-1680 cm1 but not at 3010-3090 cm-1 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • If more than one structure fits the description, draw them all. Separate structures with + signs from the drop-down menu. C opy aste ChemDoodle h 51% 10:53 A 3/23/202arrow_forwardQ.3. The low resolution mass spectrum of 3-methyl-3-pentanol (see its structure below) shows m/z peaks at 102, 87, 84, and 73. Propose the structures of these peaks. OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY