pin . A long pin is now used to hold the piston in place as shown in the diagram. The cylinder is then plaạced into boiling water. The valve remains closed for the whole time. a. Does the temperature of the gas increase, decrease, or remain the same? b. Sketch this process in the PV diagram. Using arrow, indicate the direction of the process on your graph. V c. Explain why for this particular situation, it is impossible to determine the pressure of the gas as you did on question 1 (that is, by considering the free-body-diagram of the piston).
pin . A long pin is now used to hold the piston in place as shown in the diagram. The cylinder is then plaạced into boiling water. The valve remains closed for the whole time. a. Does the temperature of the gas increase, decrease, or remain the same? b. Sketch this process in the PV diagram. Using arrow, indicate the direction of the process on your graph. V c. Explain why for this particular situation, it is impossible to determine the pressure of the gas as you did on question 1 (that is, by considering the free-body-diagram of the piston).
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 66P
Related questions
Question

Transcribed Image Text:pin
6. A long pin is now used to hold the piston in place as shown in the
diagram. The cylinder is then placed into boiling water. The valve
remains closed for the whole time.
a. Does the temperature of the gas increase, decrease, or
remain the same?
b. Sketch this process in the PV diagram. Using arrow, indicate the direction of the
process on your graph.
c. Explain why for this particular situation, it is impossible to determine the pressure of
the gas as you did on question 1 (that is, by considering the free-body-diagram of
the piston).
Expert Solution
Step 1
Given :
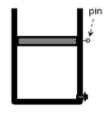
A long pin holds the piston in place (such that the position of the piston is fixed in place), and now the entire setup is placed in boiling water.
a) What happens to the temperature of the gas?
b) Draw a PV diagram, for this process described.
c) Explain why in this situation, it is impossible to determine the pressure of the gas.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning